Posts Tagged ‘Coronavirus’
What Are We Doing to Fight COVID-19? By Our Student Pharmacist, Ryan Peterson.
We are currently living in times of great uncertainty and the ideal approach to treating COVID-19 has been hotly debated. Although several therapies have been touted in the media, none have been proven effective. The decision to use a drug is based principally on observational and anecdotal reports or extrapolated from indirect evidence. Most patients who test positive can be treated at home with rest and management of symptoms. Several experimental therapies have been tried on patients with moderate or severe disease.
Below, I will outline several of the drugs that appear to be the most promising.
Remdesivir. Remdesivir is a recently developed antiviral medication. Administered by injection, remdesivir prevents the virus from replicating.1 It has shown to be effective in treating viruses similar to COVID-19 including SARS and Middle East Respiratory Syndrome (MERS-COV). Remdesivir appears to prevent COVID-19 from replicating in a laboratory setting. However, the activity of remdesivir against COVID-19 in sick patients has yet to be determined.2 Several small case studies show promise of remdesivir as an agent against COVID-19.3,4 The cases studies are limited in that they cannot compare remdesivir to no treatment or other possible treatments. Multiple larger randomized trials are currently underway to determine if remdesivir can be used as an agent against COVID-19.
What is the risk of using remdesivir before its effects are proven? Remdesivir can cause nausea, vomiting, liver and kidney damage, and potentially organ failure. In patients with kidney disease, remdesivir can build up in the body causing the side effects to last longer than normal.1
Hydroxychloroquine/chloroquine. Much speculation exists about whether hydroxychloroquine and chloroquine, either alone or in combination with azithromycin, may help treat patients with COVID-19. Please refer to my previous post (see “What is up with hydroxychloroquine?“) where I discussed hydroxychloroquine in detail. Currently, there is not enough data to say if hydroxychloroquine or chloroquine has a role in treatment of COVID-19.2 The most recent research5 (published mid April 2020) indicates that hydroxychloroquine, either alone or with azithromycin, does not reduce the severity of the disease. In fact, patients who were given hydroxychloroquine were more than twice as likely to die and more likely to require a mechanical ventilator to breathe compared to those who did not receive hydroxychloroquine.
Convalescent plasma. Researchers are investigating convalescent plasma as a potential therapy in patients with severe or life-threatening COVID-19. After a person recovers from COVID-19 they have antibodies to the disease in their blood. Within their blood, the plasma, a golden fluid that contains nutrients, can be separated from the red blood cells. There is hope that convalescent plasma can be given to people with severe COVID-19 to provide them with the antibodies needed to fight the virus and increase their chances of recovery.6
The Food and Drug Administration (FDA) has recently expanded access and approved convalescent plasma for emergency individual use.7 Several patients in case studies showed substantial improvement after they were administered convalescent plasma. This, however, does not establish a causal effect, and whether plasma is useful in therapy remains largely unknown. Additionally, hospitals face logistical challenges finding appropriate donors and establishing testing to confirm the antibodies in the plasma have activity against COVID-19. 2,6
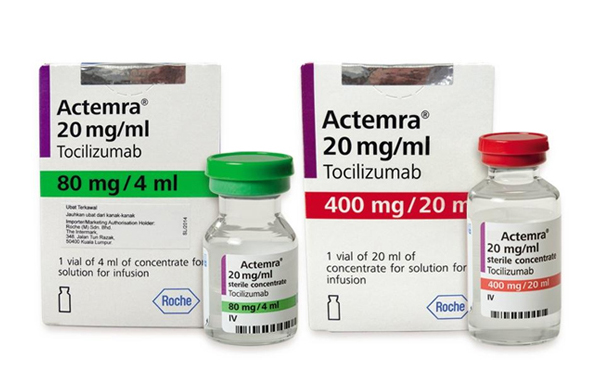
Tocilizumab. Tocilizumab inhibits the activity of Interleukin 6, a protein that affects the body’s response to infection and inflammation. Tocilizumab is approved by the FDA to treat rheumatoid arthritis and cytokine release syndrome.8 The basis for the use of tocilizumab to combat COVID-19 stems from reports of elevated Interleukin levels in patients with severe COVID-19. As with the other potential therapies, several cases indicate the positive impact of tocilizumab, but its place in therapy is still uncertain. Other Interleukin 6 inhibitors such as sarilumab and siltuximab, are also being evaluated in clinical trials.2
References
- LexiDrugs. Hydroxychloroquine. Updated March 2020. Accessed on 21 April 2020
- Coronavirus disease 2019 (COVID-19). Updated April 2020. Available at Uptodate.com. Accessedon 21 April 2020
- Lescure, F.X. et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. doi: https://doi.org/10.1016/S1473-3099(20)30200-0•
- Grein, J. et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19.N Engl J Med.doi: 10.1056/NEJMoa2007016
- Magagnoli, J. et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. (In Press) medRxiv preprint. April 2020.doi: https://doi.org/10.1101/2020.04.16.20065920
- Mayo Clinic. Convalescent plasma: Possible treatment for COVID-19? 2020. Available at: https://www.mayoclinic.org/diseases-conditions/coronavirus/expert-answers/convalescent-plasma/faq-20484383. Accessed on 21 April 2020.
- US Food and Drug Administration. Recommendations for Investigational COVID-19 Convalescent Plasma. Available at: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma. Accessed on 21 April 2020.
- LexiDrugs. Tocilizumab. Updated March 2020. Accessed on 21 April 2020.
- Michot, J.M. et al. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Onco 2020. doi: 10.1016/j.annonc.2020.03.300.
What is Up with Hydroxychloroquine? By Our Student Pharmacist, Ryan Peterson.
With the ongoing COVID-19 pandemic, there has been much speculation about the use of hydroxychloroquine from public officials and in the media. Therefore, with all the information floating around, it is important to separate fact from speculation.
What is hydroxychloroquine?
First approved for medical use in the United States in 1955, hydroxychloroquine is used for the short-term treatment of malaria and the long-term treatment of various autoimmune diseases such as lupus, rheumatoid arthritis, and dermatomyositis. Hydroxychloroquine is marketed under the trade name Plaquenil.
Does hydroxychloroquine work against COVID-19?
The short answer is possibly. Hydroxycholoroquine has been shown to have direct antiviral activity against SARS-CoV-2 (COVID-19) in a petri dish in a laboratory under controlled conditions. Several small clinical trials looking at whether hydroxycholorquine works have led to conflicting results. Two small studies1,2 in China found that hydroxychloroquine either alone1 or in combination with azithromycin2 appeared to be associated with a more rapid decline in viral RNA. However, another small observational study3 in patients with more severe illness did not suggest rapid viral RNA clearance after treatment with hydroxychloroquine and azithromycin. Multiple trials evaluating hydroxychloroquine for COVID-19 treatment are underway.
Has hydroxychloroquine been shown to prevent COVID-19?
No medication including hydroxychloroquine is known to be effective for preventing COVID-19. Clinical trials are ongoing to determine whether hydroxychloroquine should be used in post-exposure prophylaxis for household contact and in healthcare workers. It has been suggested that neither hydroxychloroquine nor any other medication be used for COVID-19 prophylaxis outside of clinical trials. 4
Why is there hesitation among clinicians to use hydroxychloroquine?
There are three main reasons physicians are hesitant about the use of hydroxycholoroquine in COVID -19 patients: 5
- Hydroxychloroquine is associated with significant side effects. In many patients, the potential side effects outweigh the benefits of the medication which is a major reason why the drug has not been used.
- Hydroxychloroquine is a critical drug used for the treatment of malaria and other diseases throughout in the world. A surge in the use of hydroxychloroquine or use in unapproved indications (including COVID-19) can create major drug shortages.
- While clinicians are hopeful that hydroxychloroquine will work to treat COVID-19, they do not have enough evidence at this time to say whether it does or not. Asking a patient to take a medication that can cause significant side effects and has yet to be proven beneficial is very difficult except in extreme cases.
What side effects are associated with hydroxychloroquine?
Patients taking hydroxychloroquine have reported numerous side effects.
These include:
- dizziness
- fatigue
- headache
- anxiety
- weight loss
- diarrhea
- hair and skin discoloration
- hair loss
- nausea and vomiting
Additionally, hydroxychloroquine has been associated with heart problems that make it harder to pump blood to the rest of the body and can lead to heart failure. 6
It is often recommended that before patients take hydroxychloroquine they receive genetic testing. Patients taking hydroxychloroquine with a mutation leading to a particular enzyme, G6PD, being deficient are at a higher risk to develop a condition called hemolytic anemia where the body destroys red blood cells faster than they are made.
Can I get hydroxychloroquine?
Hydroxychloroquine is a prescription only medication. The Food and Drug Administration (FDA) issued an emergency use authorization to allow the use of hydroxychloroquine and other agents in adolescents or adults hospitalized for COVID-19.7 Additionally, the Ohio Board of Pharmacy issued an emergency rule8 regarding the dispensing of hydroxychloroquine. According to the rule, new prescriptions of hydroxychloroquine are limited to a 14 day supply and must contain the patient’s diagnosis. Prescriptions written for a COVID-19 diagnosis must have documented a positive test result on the prescription. Additionally, prescriptions for either patients believed to be positive with COVID-19 or prophylactic use is strictly prohibited.
References:
- Gautret et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. International Journal of Antimicrobial Agents – In Press 17 March 2020 DOI:10.1016/j.ijantimicag.2020.105949.
- Molina JM,et al. No Evidence of Rapid Antiviral Clearance or Clinical Benefit with the Combination of Hydroxychloroquine and Azithromycin in Patients with Severe COVID-19 Infection. Medecine et Maladies Infectieuses,
- Chen J,et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). Journal of Zhejiang University, 2020.
- Coronavirus disease 2019 (COVID-19). Updated April 2020. Available at Uptodate.com. Accessed April 7, 2020.
- Cortegiani, A. et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19, Journal of Critical Care, In Press 2020. https://doi.org/10.1016/j.jcrc.2020.03.005
- LexiDrugs. Hydroxychloroquine. Updated March 2020. Accessed on April 7, 2020
- US Food and Drug Administration. https://www-fda-gov.proxy.lib.ohio-state.edu/media/136534/-download. Accessed on April 7, 2020
- State of Ohio Board of Pharmacy. ORC 4729-5-30.2 – Prescription requirements for chloroquine or hydroxychloroquine. Updated March 26, 2020.