Archive for June, 2022
Paxlovid: A New Treatment for COVID-19. By Our Student Pharmacist, Andrew P. Capozzi.
Ever since COVID-19 began to appear around the globe in 2019 and 2020, the world looked forward to a vaccine that could help prevent the infection from spreading.
While many of us are aware of the vaccine products that were made available by late 2020 and are still widely used today, such as the Pfizer, Moderna, and Johnson & Johnson vaccines, no medications that were directly designed for the treatment of the COVID-19 virus were available at the time. The only medications being tried were other antiviral medications that appeared to have some effect on the unique virus.
However, in December of 2021, the Food and Drug Administration (FDA) reviewed a new medication made by Pfizer called Paxlovid and gave it Emergency Use Authorization (EUA), making the medication available to be used during the public health emergency despite having not been fully approved by the regulatory agency yet (which can take up to several years).
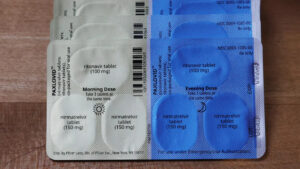
Paxlovid contains two separate antiviral medications that are packaged together. Those medications are nirmatrelvir 300 mg with ritonavir 100 mg.
The nirmatrelvir component helps to stop a key process that the COVID-19 virus requires in order to make more functioning virus particles. After treatment, the virus that is released from infected cells is not easily able to enter non-infected cells, preventing the infection from spreading easily within the body.
The ritonavir component of Paxlovid helps to boost the levels of the nirmatrelvir in the body, making the antiviral effect that much more effective.
Patients are eligible to be prescribed Paxlovid by their doctor if they meet all of the following criteria:
- Age 12 or older
- Weigh at least 88 pounds
- Had a positive COVID-19 test result
- Began experiencing symptoms within the past five days
- Are at high risk for developing severe COVID-19, meaning that patients must either:
- Have certain underlying conditions (including cancer, diabetes, obesity, or others), or
- Are age 65 or older
As more studies are carried out to prove the safety and effectiveness of the medication in other groups of people, it’s expected that the eligibility criteria may become more relaxed in the future, making the medication more accessible to others who are diagnosed with COVID-19. When being prescribed this medication, patients will often be requested to take the medication by mouth twice daily for five days.
Some important side effects that patients may experience include diarrhea and muscle pain.
When starting Paxlovid, it’s important for patients to discuss with their doctor any other medications or supplements that they may be taking as this medication is known to interact with several others, such as some anti-seizure medications, anti-cancer medications, mental health medications, heart health medications, and more.
Interestingly, other companies are also trying to develop COVID-19 treatments, such as the pharmaceutical manufacturer Merck with the development of molnupiravir, which is also available for use in having been given EUA by the FDA.
As the world continues to emerge from the COVID-19 pandemic, the pharmaceutical industry remains dedicated to developing medications that combat the virus for those that are infected and promote a healthy recovery.
Resources:
HHS Office of the Assistant Secretary for Preparedness and Response (ASPR). Paxlovid: Emergency Use Authorization. U.S. Department of Health & Human Services. Published December 2021. Accessed June 9, 2022. https://aspr.hhs.gov:443/COVID-19/Therapeutics/Products/Paxlovid/Pages/emergency-use-authorization.aspx
National Institutes of Health (NIH). Paxlovid Drug-Drug Interactions. COVID-19 Treatment Guidelines. Published May 13, 2022. Accessed June 9, 2022. https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir–paxlovid-/paxlovid-drug-drug-interactions/
O’Shaughnessy J. Emergency Use Authorization 105. Published online December 22, 2021. Accessed June 9, 2022. https://www.covid19oralrx-hcp.com/files/FDA-APPROVED_PAXLOVID-EUA-and-MATERIALS_EUA-Letter.pdf
Pfizer. Paxlovid: Fact Sheet for Healthcare Providers. Revised online April 14, 2022. Accessed June 9, 2022. https://www.covid19oralrx-hcp.com/files/Clean_Emergency-Use_Full-Prescribing-Info_HCP-Fact-Sheet-COVID-19-oral-antiviral.pdf
Pfizer. Paxlovid: Fact Sheet for Patients, Parents, and Caregivers. Revised online April 14, 2022. Accessed June 9, 2022. https://www.covid19oralrx-hcp.com/files/Clean_EUA-Fact-Sheet-for-Patients,-Parents,-and-Caregivers-COVID-19-Oral-Antiviral.pdf
Please Welcome Dennis Zhang Our Student Pharmacist from The Ohio State University’s College of Pharmacy.
This month, we are joined at Plain City Druggist by Xiancong (Dennis) Zhang, a fourth-year pharmacy student from The Ohio State University’s College of Pharmacy.
Dennis will graduate in May 2023 with his PharmD degree and will then take the test to become a registered pharmacist. Dennis will be with Tayler and the gang here in Plain City throughout June, so please stop by and meet him while he is here.
Here is what Dennis tells us about himself:
At home I grow plants, cook, and spend some time with my parakeets.
Please Welcome Andrew Capozzi Our Student Pharmacist for June at Happy Druggist on Karl Road.
This month, we are joined at Happy Druggist on Karl Road by Andrew Capozzi, a fourth-year pharmacy student from The Ohio State University’s College of Pharmacy.
Andrew will graduate in May 2023 with his PharmD degree and will then take the test to become a registered pharmacist. Andrew will be with Kristie and the staff on Karl Road throughout June, so please stop by and meet him while he is in the pharmacy.
Here is what Andrew tells us about himself:
My name is Andrew Capozzi and I’m currently a fourth year pharmacy student within The Ohio State University’s College of Pharmacy program, planning on graduating in May of 2023, and a Medication Safety Intern at The Ohio State University Wexner Medical Center.
Concurrently with my pharmacy studies, I am additionally completing an Interdisciplinary Specialization in Global Health and Certificate in Global One Health. As a true Buckeye at heart, prior to pharmacy school, I completed my undergraduate career at Ohio State, obtaining my Bachelor’s Degree with Honors in Pharmaceutical Sciences alongside minors in German, Epidemiology, and Substance Misuse & Addiction.
Before starting my college curriculum, I was raised in southwest Ohio in a small town outside of Dayton.
My interest in pursuing a career in pharmacy began in an unconventional manner during my adolescence. After having learned to read, I would often be found practicing my literary skills on prescription and OTC bottle labels, often in lieu of traditional books or literature. I would proceed to inquire with my parents about what made each medication different, the dangers of misuse, and why variations of treatment exist for the same indications.
As my academic career progressed into high school, I was enrolled in the Project Lead the Way biomedical science curriculum that provides students with an introduction to scientific studies such as: human medicine, physiology, genetics, microbiology, and public health. Through the stratified courses, I was able to gain a fundamental introduction to pharmacology that furthered my interest in pursuing a career in the related field.
All of my experiences, professional and academic, have cultivated an interest in non-traditional pharmacy practice. My passions within the profession are primarily rooted in ambulatory care and industry settings, with concentrations of commercial, regulatory affairs, pharmacovigilance, medication safety, immunization services, and global health promotion being of exceptional interest. Upon graduation, I hope to pursue a fellowship and a future career within industry that can combine my passions for pharmacovigilance, public health, and global operations.
During my time in pharmacy school, I maximized my engagement with various student organizations that represent my unique interests and professional development within the field. Spanning P1 to P3 years, I served as Pledge Educator and Regent of Kappa Psi, Vice President of Phi Lambda Sigma, Director of Programming for IPhO, the Commercial Team Lead for IPhO’s VIP Case Competition, the Ohio State Ambassador and Student Coordinator of the 2020 International Pharmacy Experience, IPSF Committee Chair of APhA-ASP, a mentor within the College of Pharmacy’s Mentorship Program, an ambassador for Generation Rx, and a member of SSHP.
Outside of pharmacy, I have further academic interests in public health and global politics, reinforced during my time in having been a member of Ohio State’s internationally-competing Model United Nations team during my undergraduate career.
However, my greatest passion is for travel, having now been to 32 different states and 14 different countries.
I am ecstatic to be spending the month at Happy Druggist and to gain experience in the field of independent pharmacy practice!